Bromine Donor
#1

Posted 11 September 2007 - 01:29 PM
#2

Posted 11 September 2007 - 05:08 PM
Hey guys, does anyone know how to make a bromine donor , other then CBr4?
Bromine is used in hot tubs/spas in the same manner as chlorine to control bacteria or algea growth. It can be bought as small pucks I think. Not sure what else they contain though.
#3

Posted 12 September 2007 - 07:32 AM
do you have any particular compound in mind?
Liquid Bromine is quite easy to make from this, although you will need to take several safety precautions when handling it, primarily Don`t breathe it or get any of it on your skin or eyes!
#4

Posted 12 September 2007 - 11:51 AM
I thought to use : KBrO3, CuBr, Lactose and a bromine donor. I can make some CBr4 but i dont have enough bromine and it is a pain in the ass to produce it in large quantities. So I am looking for alternatives. I already tried to brominate Iditol and shellac, but i didnt try those yet.
So as i was saying i need something like PVC but with bromine instead of chlorine. So neither KBr nor Br2(of course) wont do the trick
#5

Posted 12 September 2007 - 11:54 AM
and also that Bromates are Much more sensitive than Chlorates too.
#6

Posted 12 September 2007 - 12:13 PM
Bromine and its compounds are not particularly great...
Regards,
Mike
#7

Posted 12 September 2007 - 06:48 PM
Am I correct?
#8

Posted 12 September 2007 - 07:05 PM
for God`s sake Please be VERY careful and do all your homework before even Trying to do anything with Fluorine / Fluorides, it really isn`t something amateurs should mess with, it`s insanely toxic!
please trust me on this one!
#9

Posted 12 September 2007 - 09:48 PM
I tend to stay away from most chemicals that I know will kill me and I recommend the same.
I am not sure how bromine would do as a colour saturator but so far I have not heard anything about it.
If he is making a reference to bromates, then as far as I know they have only been used in small experiments and not really applied in pyrotechnics because like chlorates, they react quickly to most acids.
#10

Posted 13 September 2007 - 02:49 AM
however the temperature here is too high and the blue is somewhat washed out. The Bromate + CuBr + S is good but it is somewhat suicidal. the lactose should solve that problem and the bromine donor should do miracles on it.
The sensitivity issue is also known, so is the toxicity. You know that sodium nitrate is also a possible carcinogen ... those possible stuff tend to be overtaken . If you don't eat it daily u'll be just fine.
MDH :"If he is making a reference to bromates, then as far as I know they have only been used in small experiments and not really applied in pyrotechnics because like chlorates, they react quickly to most acids." you know that CuCl also isnt used in pyrotechnics but CuO that is a worse coloring agent is used ... you know why? price .. it is way cheaper and easier to handle in massive amounts. Chlorate isnt used due to safety in mass production .. and perbromate .. well how can i say it gently .. costs millions. so...
Fluorine. never heard of it being a good coloring agent ... well i guess no one lived to tell
#11

Posted 13 September 2007 - 09:45 PM
As far as a good fluorine based oxidiser, try teflon.
Edited by Mumbles, 13 September 2007 - 09:46 PM.
#12

Posted 13 September 2007 - 11:50 PM
And mumbles where do you get the specra's from? id like to see those figures, i mean of various halides.. not just copper
#13

Posted 17 September 2007 - 11:18 PM
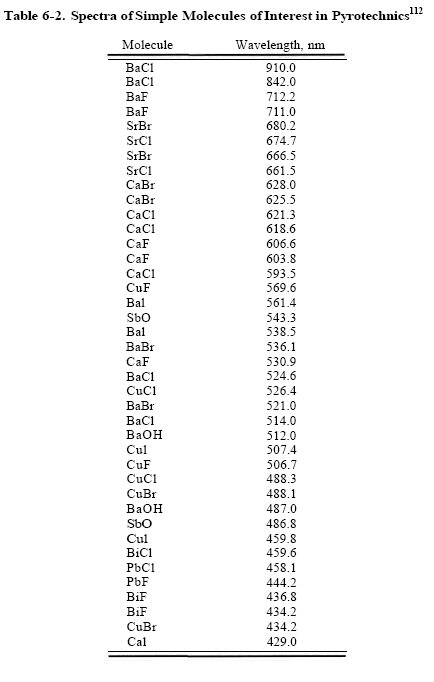
#14

Posted 18 September 2007 - 07:49 AM
The PbCl looks interesting. did anyone tried to add some PbCl2 into a cholorate based composition?
Edited by hashashan, 18 September 2007 - 07:57 AM.
#15

Posted 18 September 2007 - 01:57 PM
1 user(s) are reading this topic
0 members, 1 guests, 0 anonymous users













